Radiocarbon dating of the Voynich Manuscript
Measure what is measurable and make measurable what is not.
— Galileo Galilei
AMS (accelerator mass spectrometry) is used to determine the age of milligram samples of historical objects. This equipment was used at the University of Arizona (U of A) radiocarbon dating laboratory to date four of the Voynich Manuscript’s (VM) parchment pages. This analysis gave a mean of 516±18 BP years (before present, 1950) or 1421±8.5 years AD, indicating that the 95% confidence interval for the date when the parchment was made is probably between the years 1404-1438, (mean ±2sd). Radiocarbon dating therefore excludes Roger Bacon or anyone living before the beginning of the 15th century as a potential author of this manuscript. It indicates the date when the parchment was made, but not the date when the VM was written. I have written two previous articles on statistics and the interpretation of results obtained from AMS measurements of 14C(I)(II). This article discusses the precision and accuracy of the U of A’s measurements, the systematic errors observed when prestigious labs date the same object and the conversion of 14C BP years to AD years using the tree ring curve.
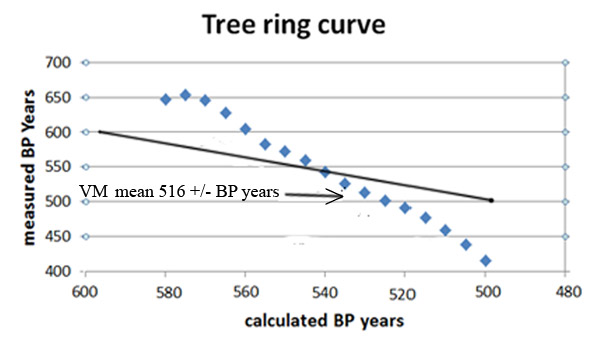
The graph below is a plot of 2004 tree ring data points (blue dots) used to date the VM. The y-axis is the measured BP age and the x-axis is the calculated BP age (x = 1950 - tree ring age) in 5-year intervals(III). The solid black line represents the decay of 14C without variations in the concentration of atmospheric 14C. The measured BP age ranges from 646 to 415 (231) years is condensed to 70 years (570-500). The slope of the measured tree ring curve is approximately linear with a gradient of ~3, the black line has a gradient of 1.
The AMS radiocarbon dating procedure uses milligram samples of parchment that are subjected to a complex series of washing chemical reactions and finally the differential analysis of three carbon isotopes using the AMS mass spectrometer. All analytical procedures are subject to error and in the case of radiocarbon dating, the 1sd counting error (counting statistic) used by most laboratories, reflects only the performance of the AMS instrumentation, not the total analytical error(IV). The counting error should be combined with the errors from every procedure in the dating process. As this is not feasible, it is recommended that the 1sd counting error of 18 in BP years be doubled in order to obtain a more reliable estimate of the total imprecision of the 14C dating of the VM’s parchment(V). A more realistic value for the U of A’s mean value would be 516±36 BP years, or 1424±13 AD years, instead of 1421±8.5 AD years.
The U of A calculated the 95% confidence interval for their mean of 1421±8.5 by doubling their 1sd standard deviation to 17 years and adding or subtracting 17 from their mean of 1421 to give a range of 1404-1438 years. This calculation is only valid if the tree ring curve is linear over the ±2sd range. It is therefore preferable to double the 1sd standard deviation of 18 BP years to 36 and extrapolate a 95% confidence interval of 1406-1434 from the tree ring curve. If the 1sd standard deviation is doubled to 36 BP years, the 2sd standard deviation becomes 72 and the 95% confidence interval for the age of the parchment lies between the years 1394-1443.
The 1sd standard deviation only relates to the precision of the measurements, i.e. how closely repeated measurements on the same sample match each other. We have no idea of the accuracy of the mean, i.e. how closely this value represents the true answer. The accuracy of radiocarbon measurements depends on how the sample, prior to analysis, is cleaned(VI). This is critical if an accurate result is required. Surface contaminants, like lipids (fats contain 14C) from handling the parchment and 14C deposited from the fallout from the detonation of atomic bombs between 1950–1960, must be removed, or the parchment will appear younger than its true age. Chalk (calcium carbonate) was detected on the parchment’s surface when McCrone and Associates examined the VM’s ink and pigments(VII). As chalk contains no 14C, its presence would make the parchment appear older. The lipid and 14C contaminants were removed by using the standard procedure of extracting the parchment with solvents like acetone and hexane, followed by acid, base, acid washes with a final wash with distilled water, before drying. The acid washings should remove the surface chalk.
There are problems associated with extracting collagen from parchment, it requires slightly more sample, increases the cost of the analysis, adds complexity and time to an already very complex process and all or some of the sample may be lost. It is probably adequate to just know that due to the presence of internal contaminants like lime and fish glue the VM’s parchment date may be slightly younger than the measurements indicate.
Systematic errors may occur if a lab fails to maintain adequate quality control of their methods, equipment, radioactive standards and control materials and will affect the accuracy of the result. I do not know whether radiocarbon laboratories, like medical laboratories, are monitored by an external agency to check the reliability of their measurements. However to obtain an idea of interlaboratory accuracy with respect to radiocarbon dating using the AMS procedure, consider the results obtained when the U of A and two other prestigious radiocarbon labs, independently measured a sample from the Shroud of Turin. The results, given in radiocarbon years, prior to conversion to an AD calendar age, are: 646±31, 750±30 and 676±24 years(X). The differences in the results demonstrate the limitation of the AMS procedure. The precision reported by the three labs is similar but utterly belies the 104 year range of their means. This is best explained by the presence of systematic error on the part of one or more of the labs.
The University of Leicester’s claim that the skeleton found in a parking lot in Leicester, England belongs to Richard III has been confirmed by DNA analysis(XI). Richard III died at the battle of Bosworth in 1485. Radiocarbon dating of bone collagen from the skeleton was undertaken by two prestigious radiocarbon labs, Glasgow University and the Oxford Radiocarbon Accelerator Unit. Glasgow University reported that their bone sample dated, with a 95% probability, to lie between the years 1430-1460 and Oxford dated theirs, with the same probability, to lie between 1412-1449(XII). The difference in the two mean values is ~15 years, the Glasgow mean is 40 years and Oxford 55 years earlier than Richard III’s death in 1485. Due to evidence that indicated that Richard III ate a diet rich in sea food, the dating of the bones was adjusted, with a 95% degree of confidence, to lie between the years 1450-1540 A.D. Some skeptics questioned whether the remains are those of Richard III. Not all their reasons are pertinent to this discussion, however, Professor Michael Hicks, head of the History Department of the University of Winchester, queried the radiocarbon dating results, stating that ‘the method is imprecise, it will give you the era, but nothing more’(XIII). The results from both Glasgow’s and Oxford’s radiocarbon labs have about the same degree of precision as the U of A’s results for the VM. They extrapolated their data, like the VM’s data from the same region of the tree ring curve. Carbon-14 from collagen was used to date both Richard III’s bones and the VM’s parchment, however the pretreatment of the respective samples was different. Is the 15-year difference in the two dates obtained for Richard III’s bones, due to systematic error and did a diet rich in seafood account for all of the 40-55 year difference between the 14C measurements and the date of Richard III’s death? Considering the size of the samples analyzed and the complexity of the analytical procedures, the errors are small but not entirely insignificant. As Professor Hicks states, radiocarbon dating provides only the era but nothing more. This is not only true for Richard III’s remains but also for the Voynich manuscript.
Carbon-14 decays exponentially with a half-life of 5730±40 years. It is generated at a fairly constant rate in the earth’s upper atmosphere by the collision of neutrons from cosmic rays with nitrogen atoms. The rate varies slightly due to changes in the flux of cosmic rays and variations in the earth’s magnetic field, thus causing small variations in the concentration of atmospheric 14C. For this reason, a radiocarbon measurement in the form of a 1sd range is converted to an AD calendar range using a radiocarbon tree ring calibration curve. This curve represents 14C measurements in BP years, using the AMS system, of tree rings plotted verses their age in AD years. A number of labs contribute to this data and it is frequently updated and improved. The graph below shows the region of the tree ring curve used to convert the U of A’s 14C measurements in BP years to AD years and describes the process.
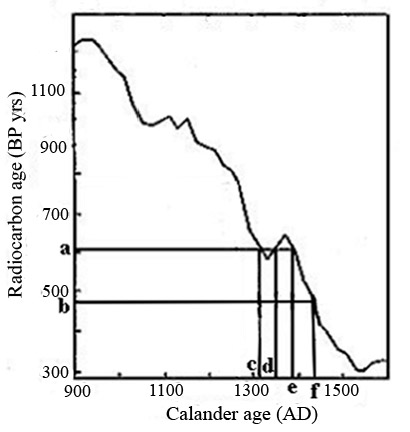
- In order to retain the 1sd counting error, a range, a b (radiocarbon measurement ±1sd), is used.
- The spike in the tree ring curve around 1360 causes complexity, the projected line from ‘a’ intersecting the curve in three places. This results in three possible AD calendar ranges, c f, d f and e f, i.e. three results.
- Due to the rapid change in the slope of the tree ring curve from ~1400 to 1500 A.D., the 1sd A.D. calendar ranges, d f and e f, are much smaller than the range, a b, giving the appearance of greater precision. A result, with the same 1sd value, but falling on a flatter part of the curve would appear to be less precise.
- Any change, like doubling the precision, would change the intercepts on the curve and therefore change the result. The same is true, for corrections in accuracy.
- Each tree ring value (sampled every five years) comes with its own 1sd error. This error is combined mathematically with the 1sd of the parchment measurement when determining the range in AD years for the parchment.
The problems in radiocarbon dating resulting from systematic error could be overcome by using the following method: In the archives of Milan, Florence and Rome are many dated parchment documents. If a selection that spans the 14th and 15th centuries were analyzed, a parchment/A.D. curve could be generated. An accurate date for a VM page could be derived from this curve if the same lab made all the measurements. It would also be interesting to compare this parchment curve with the tree ring curve.
Table I records the 14C results of four pages from the VM, obtained from an informative article on this topic by Rene Zandbergen(XIV). The U of A has presented their data at various meetings, but has never published a detailed description of their analytical methods and results.
| Folio No. | Type | Size | Parchment | BP age* | SD |
|---|---|---|---|---|---|
| f.8 | Botanical | Standard | Thin | 490 | ±37 |
| f.26 | Botanical | Standard | Thin | 506 | ±35 |
| f.47 | Botanical | Standard | Thick | 514 | ±35 |
| f.68 | astronomical | Foldout | ? | 550 | ±35 |
| Mean | 516 | ±18 |
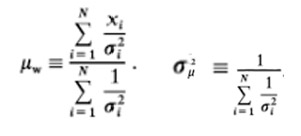
The VM is unique—not all the pages are the same size and the parchment varies in thickness. This suggests that its author may have initially recorded ideas on scraps of parchment that were later bound into a book. If folio 68’s parchment is considered to be an outlier, it may have an earlier date than the other three folios. Table II gives the dates of the individual samples in AD years and shows that the dates of the VM’s 116 pages may vary by ~21 years (1428-1407). The date when the VM’s author started this book may be later than the latest date recorded, or around that date if he had to wait for scraps of parchment to become available.
| Folio No. | Years BP years ±1σ | AD age ±1σ |
|---|---|---|
| f.8 | 490±37 | 1428±14 |
| f.26 | 506±35 | 1424±15 |
| f.47 | 514±35 | 1421±17 |
| f.68 | 550±35 | 1407±14.5 |
Many scientists quote the British Prime minister, Benjamin Disraeli’s statement: ‘there are lies, damn lies and then there is statistics’ to express their frustration with statistics. You must judge for yourselves whether applying statistics to a limited data set is justified.
The intention of this article is to evaluate possible errors in the 14C dating of the VM. The U of A performs careful, reliable work, however it is not unreasonable to suggest that their quoted standard deviations be increased to include the total errors in the dating process or that the presence of lime bonded to the collagen will cause the age of the parchment to appear slightly older than it actually is. If the VM was already in the form of a book when first used, doubling the standard deviation will date the parchment, with 95% degree of confidence to lie between the years 1394-1443. If you consider that folio 68, is an outlier and the author used scraps of parchment to record ideas before having them bound into a book, then the dating of the VM, using the same adjustment, will have a 95% confidence interval of 1391-1483. If the presence of lime is also considered, the VM’s parchment may date closer to the middle of the 15th century than the U of A’s 95% confidence interval of 1404-1438 suggests. To date, the age of the VM’s drawings and script requires the assessment of art historians.
- ↑ back Sherwood, E., Radiocarbon dating statistics in reference to the Voynich Manuscript, http://www.edithsherwood.com/radiocarbon_dating_statistics/.
- ↑ back Sherwood, E., Radiocarbon dating statistics in reference to the Voynich Manuscript Part II, http://www.edithsherwood.com/radiocarbon_dating_statistics_part2/.
- ↑ back P.J.Reimer et.al., Radiocarbon Vol. 46, 2004.
- ↑ back E Marian Scott, Gordon T Cook and Philip Naysmith, Error and Uncertainty in Radiocarbon Procedures, Radiocarbon, Vol 49, Nr 2, 2007, p 427–440.
- ↑ back E Marian Scott, Gordon T Cook and Philip Naysmith, ibid.
- ↑ back Fiona Brock, Radiocarbon dating of Historic Parchment, http://journals.uair.arizona.edu/index.php/radiocarbon/article/viewFile/16294/pdf.
- ↑ back McCrone and Associates, http://beinecke.library.yale.edu/sites/default/files/voynich_analysis.pdf.
- ↑ back Sealy et.al, http://www.sciencedirect.com/science/article/pii/S0305440314001460.
- ↑ back Fiona Brock, ibid.
- ↑ back P.E. Damon et.al., The Radiocarbon dating of the Shroud of Turin, http://www.shroud.com/nature.htm.
- ↑ back University of Leicester, Richard III Dna results, http://www.le.ac.uk/richardiii/science/resultsofdna.html.
- ↑ back University of Leicester, http://www.le.ac.uk/richardiii/science/carbondating.html.
- ↑ back BBC History Magazine, http://www.historyextra.com/news/was-skeleton-found-leicester-car-park-really-richard-iii.
- ↑ back Rene Zandbergen, http://www.voynich.nu/extra/carbon.html.
- ↑ back Courtney Taylor http://statistics.about.com/od/Descriptive-Statistics/a/How-Do-We-Determine-What-Is-An-Outlier.htm.
Acknowledgements: I thank my husband John Sherwood for acting as a critic for this article and as always my daughter Erica for her much appreciated work on my web site.